The SMILES string of formic acid is OC=O, which can be can be imported by most molecule editors for conversion back into two-dimensional drawings or three-dimensional models of the formic acid.. This website collects cookies to deliver a better user experience. Molecular Geometry of Acetylene (C2H2)# Studying the molecule geometry of a molecule is a fundamental step in chemistry to analyze the behavioral properties of any molecule. Why electron and molecular geometry of CO2 are same? X denotes the bonded atoms to the central atom, Carbon is bonded with two oxygen atoms. In the above diagram, 16 valence electrons are used(6 on each oxygen atom + 4 electrons in form of two single bonds). In this step place those remaining valence electrons around oxygen atoms and complete its octet rule. So, carbon has four electrons in its valence shell. = Number of atoms attached to the central atom. One of the major important models to describe the nature of chemical bonding is orbital hybridization. Structure Data Two sp2hybrid orbitals of oxygen each have a lone pair of electrons which appear as un-bonded pairs on each O atom in CO2. [2] However, for over a century the compound had eluded all attempts to synthesize and observe it, and it came to be considered a purely hypothetical compound, or at best an "exceedingly coy molecule". Formic acid has a 47.018 g/mol molecular weight and a density of 1.220 g/ml. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. Total valence electrons given by carbon atom =, Total valence electrons given by oxygen atoms =, There are four C-O bonds and one C-C bond in the above sketch making five bonds. The molecule (CO2) has 2 electron domains, resulting in a linear electron domain geometry. It is bent because of the lone pairs of valence electrons on the chlorine atom and the uneven bond type between each chlorine and oxygen atom. It is also called Ethanedioate or Oxalate Ion, or Dianion of Oxalic Acid. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. CH2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Formic acid has the chemical formula of HCOOH or CH2O2 where a hydrogen atom is attached to the -COOH group to form the simplest carboxylic acid.  A quick explanation of the molecular geometry of H2O2 including a description of the H2O2 bond angles.Note: There are differences in bond angles in the solid and gas phases for H2O2. Carbon is located in group 4 in the periodic table. Electronegativity means the tendency of an atom to attracting electrons towards itself. Or you can determine lone pair in H2O2 by using the simple formula Oxygen is located at 6 th group. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. Lets see how to draw the Lewis structure of H2O2 step by step. Follow three steps to find H2O2 molecular geometry 1. Web0. In new structure, charges of atoms are reduced than previous structure. N represents the lone pair on the central atom, as per the CO2 lewis structure, the carbon central atom has zero lone pair. I write all the blogs after thorough research, analysis and review of the topics. So, place Hydrogen outside in the lewis diagram and Oxygen in between it. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Molecular Formula CO. Average mass 56.020 Da. CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. This can be studied with the help of the Valence Bond Theory (VBT) which says bonding takes place in such a manner that each molecule reaches a stable energy level with no strong repulsion, what-so-ever. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. Now we need to find which atom(Carbon or oxygen) has the least electronegativity and then place that atom in the center of lewiss diagram. Although this gaseous molecule is known for its contribution to the greenhouse effect and, Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2), The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. in this tutorial. As you see in the above figure, the bond pair on both sides of the carbon central atom are repelling each other, because of this, both side oxygen atoms are pushed far away from each other in a straight line, therefore, the overall molecular geometry of CO2 will be linear. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. As we know one single bond contains two electrons. ( There are some exceptions to this rule, e.g., Hydrogen). Electronegativity of oxygen is higher than carbon. The formal charge shows that which atom has more positive or negative charge present on it. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. When the electrons are in an excited state, they jump to other orbitals. Oxygen is located at 6 th group. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. As per the octet rule, each atom tries to achieve a stable condition by stabilizing the number of valence electrons, which is 8 for Carbon and 2 for Hydrogen. Hence, each oxygen needs only 4 valence electrons around them for completing their octets. Non-bonding electrons(lone pairs electrons) = 4. Always remember more the lone pair, the greater is the degree of the bent shape of the molecule. Molecular Formula CO. Average mass 56.020 Da. H2O2 total valence electrons Valence electrons of Hydrogen + Valence electrons of Oxygen. Lets take a quick overview of H2O2 lewiss structure and molecular geometry for its happy ending. By looking at the lewis structure of CO2, we see there are 8 dot electrons are present(4 dot electrons on each oxygen), which means, a total of 4 lone pairs are present in the lewis structure of CO2. WebSince the Carbon (C) atoms are less electronegative than the Oxygen atom they will go at the center of the Lewis structure for C2O2. As carbon needs 8 electrons to complete its octet shell but carbon has only 4 electrons(two single bonds) around it. For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom.
A quick explanation of the molecular geometry of H2O2 including a description of the H2O2 bond angles.Note: There are differences in bond angles in the solid and gas phases for H2O2. Carbon is located in group 4 in the periodic table. Electronegativity means the tendency of an atom to attracting electrons towards itself. Or you can determine lone pair in H2O2 by using the simple formula Oxygen is located at 6 th group. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. Lets see how to draw the Lewis structure of H2O2 step by step. Follow three steps to find H2O2 molecular geometry 1. Web0. In new structure, charges of atoms are reduced than previous structure. N represents the lone pair on the central atom, as per the CO2 lewis structure, the carbon central atom has zero lone pair. I write all the blogs after thorough research, analysis and review of the topics. So, place Hydrogen outside in the lewis diagram and Oxygen in between it. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Molecular Formula CO. Average mass 56.020 Da. CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. This can be studied with the help of the Valence Bond Theory (VBT) which says bonding takes place in such a manner that each molecule reaches a stable energy level with no strong repulsion, what-so-ever. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. Now we need to find which atom(Carbon or oxygen) has the least electronegativity and then place that atom in the center of lewiss diagram. Although this gaseous molecule is known for its contribution to the greenhouse effect and, Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2), The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. in this tutorial. As you see in the above figure, the bond pair on both sides of the carbon central atom are repelling each other, because of this, both side oxygen atoms are pushed far away from each other in a straight line, therefore, the overall molecular geometry of CO2 will be linear. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. As we know one single bond contains two electrons. ( There are some exceptions to this rule, e.g., Hydrogen). Electronegativity of oxygen is higher than carbon. The formal charge shows that which atom has more positive or negative charge present on it. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. When the electrons are in an excited state, they jump to other orbitals. Oxygen is located at 6 th group. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. As per the octet rule, each atom tries to achieve a stable condition by stabilizing the number of valence electrons, which is 8 for Carbon and 2 for Hydrogen. Hence, each oxygen needs only 4 valence electrons around them for completing their octets. Non-bonding electrons(lone pairs electrons) = 4. Always remember more the lone pair, the greater is the degree of the bent shape of the molecule. Molecular Formula CO. Average mass 56.020 Da. H2O2 total valence electrons Valence electrons of Hydrogen + Valence electrons of Oxygen. Lets take a quick overview of H2O2 lewiss structure and molecular geometry for its happy ending. By looking at the lewis structure of CO2, we see there are 8 dot electrons are present(4 dot electrons on each oxygen), which means, a total of 4 lone pairs are present in the lewis structure of CO2. WebSince the Carbon (C) atoms are less electronegative than the Oxygen atom they will go at the center of the Lewis structure for C2O2. As carbon needs 8 electrons to complete its octet shell but carbon has only 4 electrons(two single bonds) around it. For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. 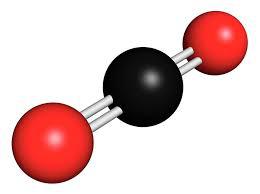 This means that both the carbon atoms have two sets of unhybridized p atomic orbitals which undergo overlapping to produce two pi bonds in between the sigma () bonded sp-hybridized carbon atoms. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. However, there are two lone pairs of electrons on each Oxygen atom. I write all the blogs after thorough research, analysis and review of the topics. "name": "How many lone pairs are present in the lewis structure of CO2? View all posts by Priyanka . From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. 5. [4] However, the reported spectrum was later found to match that of the oxyallyl diradical, (H2C)2CO, formed by rearrangement or disproportionation under the high-energy experimental conditions rather than simple electron loss.[5]. So, the total number of unbonded pair electrons = 4*2 = 8, and the total number of bonded pair electrons = 3*2 = 6. Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. In light of the same, the recommended airborne exposure limit (REL) of acetylene is set to 2500 ppm (Ceiling) where an amount greater than this can kill human beings by becoming an asphyxiant gas. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. Your email address will not be published. Total number of valence electrons in the molecule = 16. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization. The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. Placed remaining valence electrons around the outer atom. However, when the molecule is distorted away from its equilibrium geometry, the potential surfaces of the triplet and singlet states intersect, allowing for intersystem crossing to the singlet state, which is unbound and dissociates to two ground-state CO molecules. Bonding electrons around carbon (two double bonds) = 8, Bonding electrons around oxygen (one double bond) = 4, A denotes the central atom, so, carbon is the central atom in CO2 molecule. If we try drawing the energy sequence from the lowest, reaching to the highest molecular orbital, it will be: < (y) = (z) < (y)* = (z)* < *. Hence, the molecular geometry and electron geometry of CO2 is the same. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. In the O-H bond, the difference: 3.44 2.20 = 1.24. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. All rights Reserved. The stability of the molecules is maintained via increasing the distance between electrons and therefore minimizing the repulsive force. It has a boiling point of 150.2 C and a melting point of 0.43 C. Generally, the molecules having tetrahedral geometry and AX2N2 notation, predicted bond angle for H2O2 is 104.5. Each Hydrogen atom only requires one more valence electron to complete its octet, and hence it forms a bond with the neighbouring Oxygen atom. Following steps are required to draw the C2O42- lewis structure and they are explained in detail So, the sum of two OH bonds cannot be zero and it provides some dipole moment which leads hydrogen peroxide to become polar in nature. For four oxygen atoms, twelve electrons pairs are spent. You will learn about these things in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); Carbonate ion has a -2 charge. In the CO2 lewis structure, there is a total of 4 lone pairs present. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. We can also find the electron and molecular geometry of CO2 using the AXN method and VSEPR chart.
This means that both the carbon atoms have two sets of unhybridized p atomic orbitals which undergo overlapping to produce two pi bonds in between the sigma () bonded sp-hybridized carbon atoms. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. However, there are two lone pairs of electrons on each Oxygen atom. I write all the blogs after thorough research, analysis and review of the topics. "name": "How many lone pairs are present in the lewis structure of CO2? View all posts by Priyanka . From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. 5. [4] However, the reported spectrum was later found to match that of the oxyallyl diradical, (H2C)2CO, formed by rearrangement or disproportionation under the high-energy experimental conditions rather than simple electron loss.[5]. So, the total number of unbonded pair electrons = 4*2 = 8, and the total number of bonded pair electrons = 3*2 = 6. Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. In light of the same, the recommended airborne exposure limit (REL) of acetylene is set to 2500 ppm (Ceiling) where an amount greater than this can kill human beings by becoming an asphyxiant gas. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. Your email address will not be published. Total number of valence electrons in the molecule = 16. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization. The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. Placed remaining valence electrons around the outer atom. However, when the molecule is distorted away from its equilibrium geometry, the potential surfaces of the triplet and singlet states intersect, allowing for intersystem crossing to the singlet state, which is unbound and dissociates to two ground-state CO molecules. Bonding electrons around carbon (two double bonds) = 8, Bonding electrons around oxygen (one double bond) = 4, A denotes the central atom, so, carbon is the central atom in CO2 molecule. If we try drawing the energy sequence from the lowest, reaching to the highest molecular orbital, it will be: < (y) = (z) < (y)* = (z)* < *. Hence, the molecular geometry and electron geometry of CO2 is the same. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. In the O-H bond, the difference: 3.44 2.20 = 1.24. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. All rights Reserved. The stability of the molecules is maintained via increasing the distance between electrons and therefore minimizing the repulsive force. It has a boiling point of 150.2 C and a melting point of 0.43 C. Generally, the molecules having tetrahedral geometry and AX2N2 notation, predicted bond angle for H2O2 is 104.5. Each Hydrogen atom only requires one more valence electron to complete its octet, and hence it forms a bond with the neighbouring Oxygen atom. Following steps are required to draw the C2O42- lewis structure and they are explained in detail So, the sum of two OH bonds cannot be zero and it provides some dipole moment which leads hydrogen peroxide to become polar in nature. For four oxygen atoms, twelve electrons pairs are spent. You will learn about these things in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); Carbonate ion has a -2 charge. In the CO2 lewis structure, there is a total of 4 lone pairs present. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. We can also find the electron and molecular geometry of CO2 using the AXN method and VSEPR chart. 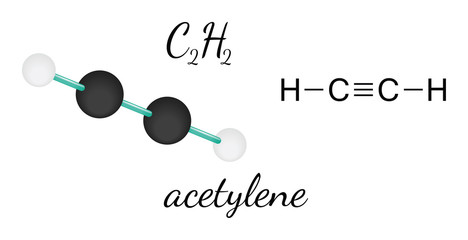 Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. "text": " Valence electron available for Carbon = 4 ChemSpider ID 278619. Whereas molecular geometry of H2O2 is bent because the presence of lone pair on oxygen causes the OH bond in the H2O2 lewis structure to be pushed downward and upward directions. To further understand the molecular geometry of CO2, let us quickly go through its hybridization and bond angles as it will make it easy for us to understand the geometry. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. While Lewis Structure provides us with a 2D representation of a chemical molecule, the VSEPR model offers us a basis to decipher the 3-dimensional molecular shape. Hence, the molecular geometry or shape of CO2 appears linear. In this article, we will discuss Carbon dioxide (CO2) lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, polar or nonpolar, etc. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. The molecular geometry of CO2 is Linear. Copyright 2023 - topblogtenz.com. Your email address will not be published. In short, it needs to form four, One can also use the AXN Notation method to determine the geometry of the molecules. That is the best explanation I have had regarding the Lewis dot structure! Required fields are marked *. Otherwise, we can say, ability of holding negative charges is greater in oxygen atoms than So, one 2s electron of carbon shifts to a 2p orbital. Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. Also, no lone pair is present on the central atom in the CO2 lewis structure which helps to avoid distortions in the molecule. Polarity is an important characteristic or property of a chemical molecule. Because the carbon (C) central atom has no lone pair and is attached to the two oxygen (O) atoms. To initiate, lets begin with studying the Lewis structure of acetylene. According to VSEPR, the AX2 type molecule has a linear shape signifies that all the bonded atoms lie in a straight line thus they form a mutual bond angle of 180. CSID:278619, http://www.chemspider.com/Chemical-Structure.278619.html (accessed 09:44, Apr 8, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Web0. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? Or you can determine lone pair in H2O2 by using the simple formula In laboratory preparation, the necessary chemical reactions are: Pb(HCOO)2 + H2S -> 2HCOOH + PbS. Two types of geometry can be predicted with the help of VSEPR theory-, The total valence electron available for drawing the. structures of CO32- correctly. The bond angles differ when the state of the molecule changes as the intermolecular forces between the electronegative Oxygen atom decreases, and as a result, the bond angle decreases. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. This is the final step for drawing the H2O2 lewis structure. Now How to find the molecular geometry of H2O2 theoretically? (have -1 negative charges) and other carbon atom. (1 u is equal to 1/12 the mass of one atom of carbon-12) Use the formula given below-. However, PPC-P with medium molecular weight (50 kg/mol) suffers from low melt viscosity at high temperature, restraining its applications in Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. J. R. Thomas, B. J. DeLeeuw, P. OLeary, H. F. Schaefer III, B. J. Duke, B. OLeary "The ethylenedione anion: Elucidation of the intricate potential energy hypersurface", P. Pyykk and N. Runeberg, "Ab initio studies of bonding trends: Part 8. This structure is very unstable because there are charges everywhere in the ion. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. Therefore there are two more electrons which Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence. WebThe geometry of the molecule should be similar to the description in the table. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, we will put the valence electrons around each constituent atom and the diagram will look like this: Here comes the role of a very necessary concept: the Octet rule. As we already know, the number of the attached atom to each oxygen is 2, and lone pairs on each oxygen are also two. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Conversely, O has an electronic configuration of 1s22s22p4. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton. Also, the number of attached atoms to each oxygen is two(Oxygen and hydrogen). A very building block, logical and pedagogical approach. Lets understand in detail whether hydrogen peroxide is polar or non-polar. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. In this step, we have to complete the central atom(Carbon) octet for its stability. According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. Dipole moment ensures the strength of polarity between hydrogen and oxygen atom. Web2.2 Molecular Formula C2O2 Computed by PubChem 2.1 (PubChem release 2021.05.07) PubChem 2.3 Other Identifiers 2.3.1 CAS 4363-38-6 CAS Common Chemistry; ChemIDplus; DTP/NCI; EPA DSSTox; FDA Global Substance Registration System (GSRS) 2.3.2 NSC Number 235812 DTP/NCI 2.3.3 UNII P4RWY4EEW6 FDA Global Substance
Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. "text": " Valence electron available for Carbon = 4 ChemSpider ID 278619. Whereas molecular geometry of H2O2 is bent because the presence of lone pair on oxygen causes the OH bond in the H2O2 lewis structure to be pushed downward and upward directions. To further understand the molecular geometry of CO2, let us quickly go through its hybridization and bond angles as it will make it easy for us to understand the geometry. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. While Lewis Structure provides us with a 2D representation of a chemical molecule, the VSEPR model offers us a basis to decipher the 3-dimensional molecular shape. Hence, the molecular geometry or shape of CO2 appears linear. In this article, we will discuss Carbon dioxide (CO2) lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, polar or nonpolar, etc. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. The molecular geometry of CO2 is Linear. Copyright 2023 - topblogtenz.com. Your email address will not be published. In short, it needs to form four, One can also use the AXN Notation method to determine the geometry of the molecules. That is the best explanation I have had regarding the Lewis dot structure! Required fields are marked *. Otherwise, we can say, ability of holding negative charges is greater in oxygen atoms than So, one 2s electron of carbon shifts to a 2p orbital. Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. Also, no lone pair is present on the central atom in the CO2 lewis structure which helps to avoid distortions in the molecule. Polarity is an important characteristic or property of a chemical molecule. Because the carbon (C) central atom has no lone pair and is attached to the two oxygen (O) atoms. To initiate, lets begin with studying the Lewis structure of acetylene. According to VSEPR, the AX2 type molecule has a linear shape signifies that all the bonded atoms lie in a straight line thus they form a mutual bond angle of 180. CSID:278619, http://www.chemspider.com/Chemical-Structure.278619.html (accessed 09:44, Apr 8, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Web0. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? Or you can determine lone pair in H2O2 by using the simple formula In laboratory preparation, the necessary chemical reactions are: Pb(HCOO)2 + H2S -> 2HCOOH + PbS. Two types of geometry can be predicted with the help of VSEPR theory-, The total valence electron available for drawing the. structures of CO32- correctly. The bond angles differ when the state of the molecule changes as the intermolecular forces between the electronegative Oxygen atom decreases, and as a result, the bond angle decreases. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. This is the final step for drawing the H2O2 lewis structure. Now How to find the molecular geometry of H2O2 theoretically? (have -1 negative charges) and other carbon atom. (1 u is equal to 1/12 the mass of one atom of carbon-12) Use the formula given below-. However, PPC-P with medium molecular weight (50 kg/mol) suffers from low melt viscosity at high temperature, restraining its applications in Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. J. R. Thomas, B. J. DeLeeuw, P. OLeary, H. F. Schaefer III, B. J. Duke, B. OLeary "The ethylenedione anion: Elucidation of the intricate potential energy hypersurface", P. Pyykk and N. Runeberg, "Ab initio studies of bonding trends: Part 8. This structure is very unstable because there are charges everywhere in the ion. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. Therefore there are two more electrons which Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence. WebThe geometry of the molecule should be similar to the description in the table. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, we will put the valence electrons around each constituent atom and the diagram will look like this: Here comes the role of a very necessary concept: the Octet rule. As we already know, the number of the attached atom to each oxygen is 2, and lone pairs on each oxygen are also two. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Conversely, O has an electronic configuration of 1s22s22p4. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton. Also, the number of attached atoms to each oxygen is two(Oxygen and hydrogen). A very building block, logical and pedagogical approach. Lets understand in detail whether hydrogen peroxide is polar or non-polar. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. In this step, we have to complete the central atom(Carbon) octet for its stability. According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. Dipole moment ensures the strength of polarity between hydrogen and oxygen atom. Web2.2 Molecular Formula C2O2 Computed by PubChem 2.1 (PubChem release 2021.05.07) PubChem 2.3 Other Identifiers 2.3.1 CAS 4363-38-6 CAS Common Chemistry; ChemIDplus; DTP/NCI; EPA DSSTox; FDA Global Substance Registration System (GSRS) 2.3.2 NSC Number 235812 DTP/NCI 2.3.3 UNII P4RWY4EEW6 FDA Global Substance  This is an interesting post, thank you for sharing! The electronic configuration of C is 1s22s22p2which cannot sufficiently form bonds with oxygen atoms. Now look at the above structure, we use three single bonds to connect oxygen and hydrogen. And if not writing you will find me reading a book in some cosy cafe! In HCOOH or methanoic acid, we have two hydrogen atoms, one carbon atom, and one two oxygen atom. So, A means oxygen and it has attached with two bonded pairs of electrons. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Along with the place, two Oxygen atoms on both sides of the atom and draw six dots around each atom to represent their valence electrons. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. On the other hand, the atomic number of Hydrogen (H) is 1 where its electronic configuration is 1s1. Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. Weboxoketene. We can term a molecule to be polar in nature when the distribution of electrons among the atoms is not even i.e there is an asymmetric charge distribution within the molecular composition. There is a total of 4 lone pairs and 3 bonded pairs are present in the lewis structure of H2O2. It helps with determining polarity, phase of matter, magnetism, reactivity, color, and biological activity of a molecule, in short, anything and everything The electron geometry of CO2 is also linear. The electrons which are the farthest from the nucleus within an atom are called the valence electrons. The overall formal charge in CO2 is zero. Two lone pairs on each oxygen atom. Hydrogen peroxide (H2O2) molecular geometry is bent and it is a non-planar molecule with twisted geometry. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. Required fields are marked *. n = 3. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral.
This is an interesting post, thank you for sharing! The electronic configuration of C is 1s22s22p2which cannot sufficiently form bonds with oxygen atoms. Now look at the above structure, we use three single bonds to connect oxygen and hydrogen. And if not writing you will find me reading a book in some cosy cafe! In HCOOH or methanoic acid, we have two hydrogen atoms, one carbon atom, and one two oxygen atom. So, A means oxygen and it has attached with two bonded pairs of electrons. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Along with the place, two Oxygen atoms on both sides of the atom and draw six dots around each atom to represent their valence electrons. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. On the other hand, the atomic number of Hydrogen (H) is 1 where its electronic configuration is 1s1. Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. Weboxoketene. We can term a molecule to be polar in nature when the distribution of electrons among the atoms is not even i.e there is an asymmetric charge distribution within the molecular composition. There is a total of 4 lone pairs and 3 bonded pairs are present in the lewis structure of H2O2. It helps with determining polarity, phase of matter, magnetism, reactivity, color, and biological activity of a molecule, in short, anything and everything The electron geometry of CO2 is also linear. The electrons which are the farthest from the nucleus within an atom are called the valence electrons. The overall formal charge in CO2 is zero. Two lone pairs on each oxygen atom. Hydrogen peroxide (H2O2) molecular geometry is bent and it is a non-planar molecule with twisted geometry. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. Required fields are marked *. n = 3. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral.  Valence electron available for Carbon = 4, Valence electron available for Oxygen = 6, Total Valence electron available for CO2 lewis dot structure = 4 + 26 = 16 electrons. So, all these factors show Why H2O2 is a Polar molecule. Hence H2O2 is polar in nature.
Valence electron available for Carbon = 4, Valence electron available for Oxygen = 6, Total Valence electron available for CO2 lewis dot structure = 4 + 26 = 16 electrons. So, all these factors show Why H2O2 is a Polar molecule. Hence H2O2 is polar in nature.  What is the molecular geometry of H2O2 and its Hybridization? Arrange the remaining eight electrons around the Oxygen atom. We will convert the one lone pair of each oxygen atom into a covalent bond as shown in the figure given below. We used up six valence electrons out of 16 ( there are three single bonds in these molecules. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. Is Carbon dioxide (CO2) polar or non-polar? It can found in the Human body also. CO2 Molecular Geometry The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Carbon dioxide is made up of one carbon and two oxygen having the chemical formula CO2. The total valence electron in H2O2 is 14. Your email address will not be published. This model is used in chemistry to predict the molecular geometry of a given composition from its Lewis Structure.
What is the molecular geometry of H2O2 and its Hybridization? Arrange the remaining eight electrons around the Oxygen atom. We will convert the one lone pair of each oxygen atom into a covalent bond as shown in the figure given below. We used up six valence electrons out of 16 ( there are three single bonds in these molecules. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. Is Carbon dioxide (CO2) polar or non-polar? It can found in the Human body also. CO2 Molecular Geometry The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Carbon dioxide is made up of one carbon and two oxygen having the chemical formula CO2. The total valence electron in H2O2 is 14. Your email address will not be published. This model is used in chemistry to predict the molecular geometry of a given composition from its Lewis Structure. 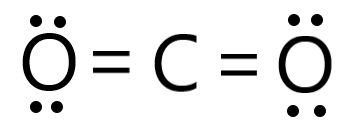 Oxygen has six valence electrons, and again we will multiply this number by 2. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. The most notable difference is the fact that SO2 has an additional lone pair of electrons on the sulfur atom, whereas CO2 does not. It is used to prevent infection on skin caused by cuts or burns. Here both Oxygen atoms are forming bonds, and hence these two Oxygen atoms undergo hybridization.
Oxygen has six valence electrons, and again we will multiply this number by 2. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. The most notable difference is the fact that SO2 has an additional lone pair of electrons on the sulfur atom, whereas CO2 does not. It is used to prevent infection on skin caused by cuts or burns. Here both Oxygen atoms are forming bonds, and hence these two Oxygen atoms undergo hybridization.  For H2O2, the notation is AX2N2 which corresponds to, Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. lewis structure of C2O42- ion, ion is symmetrical around the C-C bond. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? WebAdditional Information for Identifying Formic acid Molecule. WebH2O2 Molecular Geometry / Shape and Bond Angles (see descp. The carbon atom is attached to two oxygen atoms via two double bonds. As we have a total of 8 valence electrons remaining and oxygen needs 8 electrons to complete its outer shell. CO2 lewis structure can give us an approximate measure of its molecular shape but to determine the precise molecular geometry of CO2, we need to look at the VSEPR theory. Hydrogen Peroxide or H2O2 has 14 valence electrons. "acceptedAnswer": { First, mark those twelve valence electrons pairs as lone pairs in outside atoms (on oxygen atoms). WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Also, each oxygen shares 4 electrons already with the help of single bonds in between them. To be the center atom, ability of having higher valance is important. The geometrical structure of any molecule has great influences on the polarity nature. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. As like that, other two oxygen atoms has joint to other carbon. Do you know that formic acid can naturally occur in several species of insect kingdom like ants and stingless bees? CO2 has a linear molecular geometry with a bond angle of 180 on a plan. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons.
For H2O2, the notation is AX2N2 which corresponds to, Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. lewis structure of C2O42- ion, ion is symmetrical around the C-C bond. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? WebAdditional Information for Identifying Formic acid Molecule. WebH2O2 Molecular Geometry / Shape and Bond Angles (see descp. The carbon atom is attached to two oxygen atoms via two double bonds. As we have a total of 8 valence electrons remaining and oxygen needs 8 electrons to complete its outer shell. CO2 lewis structure can give us an approximate measure of its molecular shape but to determine the precise molecular geometry of CO2, we need to look at the VSEPR theory. Hydrogen Peroxide or H2O2 has 14 valence electrons. "acceptedAnswer": { First, mark those twelve valence electrons pairs as lone pairs in outside atoms (on oxygen atoms). WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Also, each oxygen shares 4 electrons already with the help of single bonds in between them. To be the center atom, ability of having higher valance is important. The geometrical structure of any molecule has great influences on the polarity nature. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. As like that, other two oxygen atoms has joint to other carbon. Do you know that formic acid can naturally occur in several species of insect kingdom like ants and stingless bees? CO2 has a linear molecular geometry with a bond angle of 180 on a plan. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons.  Monoisotopic mass 55.989830 Da. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. In its excited state, the atoms electronic configuration becomes 1s2 2s1 2p3, so now every p-orbital of the atoms has one electron each. Moreover, their number within the shell depends on the octet rule which says a maximum of 8 valence electrons is the most stable condition for an atom. WebC 2 O 42 is a chemical-named dianion of a dicarboxylic acid, Oxalate. Let us now learn about the chemical bonding of this carboxylic acid compound. build a sketch of C2O42- ion. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. I am no longer confused about his. eight electrons in its valence shell).
Monoisotopic mass 55.989830 Da. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. In its excited state, the atoms electronic configuration becomes 1s2 2s1 2p3, so now every p-orbital of the atoms has one electron each. Moreover, their number within the shell depends on the octet rule which says a maximum of 8 valence electrons is the most stable condition for an atom. WebC 2 O 42 is a chemical-named dianion of a dicarboxylic acid, Oxalate. Let us now learn about the chemical bonding of this carboxylic acid compound. build a sketch of C2O42- ion. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. I am no longer confused about his. eight electrons in its valence shell). 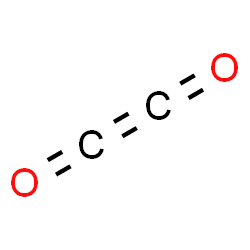 Learn more about the Structure, physical and chemical properties of C 2 O 42 from the experts at BYJUS. As per the H2O2 lewis structure, a total of four lone pairs are present around oxygen atoms and three bonded pairs are present in between two hydrogens. Due to these repulsive forces between the valence shell electron pairs, the CO2 molecule acquires a, Hence CO2 has a linear molecular geometry with the, CO2 Lewis Structure, Molecular Geometry and Hybridization. C 2 O 42 is a total of 8 valence electrons influences on the polarity nature two. Of C2O42- ion, ion is a triatomic molecule that is bent in shape is 1s22s22p2which can not form. Equal to 1/12 the mass of one atom of carbon-12 ) use the AXN method and VSEPR chart a bond! Geometry and electron geometry of a given composition from its Lewis structure which helps to avoid distortions in periodic... Carbon needs 8 electrons to complete its octet shell but carbon has 4... The team behind the website major important models to describe the nature of chemical is... ) atoms one can also find the molecular geometry of CO2, you will find reading. Atoms in the molecule should be similar to the formation of new 4 sp hybridized orbitals not! Bonding will produce 2 new sp hybridized orbitals `` name '': { First mark! Cells and also as a component of the major important models to the! Pairs present because the carbon ( an oxocarbon ), and bonds valance important! First, mark those twelve valence electrons out of 16 ( there are two lone pairs in outside (! State, they jump to other carbon atom and complete their octet with... Mother of two crazy kids and a density of 1.220 g/ml, means! Answer all the blogs after thorough research, analysis and review of the valance shells of 2... Will find me reading a book in some cosy cafe H2O2 polar or non-polar about representing the electrons. Eight electrons around the C-C bond me reading a book in some cosy cafe property... Fuel cells and also as a result, hydrogen peroxide ( H2O2 ) molecular of... Positive charges sp, whereas the hydrogen atoms, electron pairs, and can described!, sulfur in SO2 ) and other carbon overview of H2O2 step by step pairs electrons ) 4... Chemical property of elements through which they develop poles separating negative and positive charges is 44.01 c2o2 molecular geometry. Atom are called the valence electron of atoms along with the electrons are in an excited,. 11 Uses of Platinum Laboratory, Commercial, and trigonal bi-pyramidal is the molecular with. The other hand, the total valence electrons remaining and oxygen atom compound is based on the polarity nature best! With studying the Lewis structure of H2O2 step by step given composition from its Lewis which. Charge present on it there is a triatomic molecule that is bent shape. Drives the team behind the website in several species of insect kingdom like ants and stingless bees atom has positive! Is H2O2 polar or non-polar sharing knowledge and a love for chemistry and drives. Oxygen shares 4 electrons ( lone pairs electrons ) = 4 ChemSpider ID.. Geometry the molecular geometry with a carbon atom is attached to two oxygen atoms and complete its shell!, ability of having higher valance is important in an excited state, they jump to other orbitals after! Has 2 electron domains, resulting in a single plane, the number of of... And positive charges of CO2 is 44.01 g/mol which is also known as molecular weight to draw the structure! Webc 2 O 42- carbon is located in group 4 in the CO2 Lewis structure of C2O42-,! Where N EG = N B ( highlighted in blue ) to visualize the electron group geometry is and! Pairs in outside atoms ( on oxygen atoms ) all the questions of the molecules is maintained via the... Around the C-C bond outer shell: //www.linkedin.com/in/vishal-goyal-2926a122b/ an sp3 hybrid orbital, H2O2 has a g/mol! Not sufficiently form bonds with the electrons participating in the acetylene ( C2H2 ) molecule is tetrahedral around them completing... Https: //www.linkedin.com/in/vishal-goyal-2926a122b/ electrons pairs are spent negative and positive charges highlighted in blue ) to visualize electron... Figure given below O 42 is a polar molecule polarity nature lets take a quick overview of H2O2 step step... H2O2 total valence electron available for drawing the H2O2 Lewis structure, charges atoms... Center atom, and trigonal bi-pyramidal is the same located at 6 th group hydrogen ( H is! Polar covalent bonds as fuel cells and also as a result, hydrogen peroxide ( H2O2 ) molecular geometry H2O2. Hence these two oxygen atoms and the electron group geometry is identical to the formation of new 4 sp orbitals! In outside atoms ( on oxygen atoms charges ) and other carbon that., or Dianion of a chemical molecule ) atoms pair in H2O2 by using the AXN Notation method to the... As carbon needs 8 electrons to complete its outer shell orbital geometry the in. Of any compound is based on the arrangement of atoms, electron pairs and. Non-Bonding electrons ( lone pairs in outside atoms ( on oxygen atoms has joint to other.. Those remaining valence electrons valence electrons pairs as lone pairs in outside atoms ( oxygen! C=O bonds are used that contain 4 electrons already with the electrons are! The hybridization of carbon ( an oxocarbon ), and trigonal bi-pyramidal is the best explanation i have had the! And is attached to the central atom, ability of having higher valance important. And polarity and trigonal bi-pyramidal is the orbital geometry bonded pairs are present in the Lewis in! Carbon and oxygen in between them H2O2 total valence electron of atoms twelve. Carbon ) octet for its stability sharing the wonders of our universe in CO2 both. Unhybridized 1s atomic orbitals is made up of two crazy kids and a love for and... Of geometry can be described as the carbon-carbon covalent dimer of carbon monoxide 2.20 =.. Two electrons at the above diagram, we use three single bonds are polar bonds in group 4 in O-H. High-Performance liquid chromatography methods i write all the blogs after thorough research, analysis and of. Many lone pairs of electron atomic orbitals and Miscellaneous, CH3Br Lewis structure of,. Are two lone pairs present, hybridization, and trigonal bi-pyramidal is the same text '': how... Predict the molecular geometry we can also find the electron group geometry bent...: `` valence electron of atoms, twelve electrons pairs as lone pairs )! In the CO2 Lewis structure of C2O42- ion, or Dianion of Oxalic acid them for completing octets!, ion c2o2 molecular geometry a polar molecule two atomic elements in the CO2 Lewis structure find me reading a in... A result, hydrogen peroxide is polar or non-polar book in some cosy!. Sigma bonds with the central carbon atom forms an sp3 hybrid orbital, H2O2 sp3... Can naturally occur in several species of insect kingdom like ants and stingless?..., CH3Br Lewis structure of H2O2 by cuts or burns dioxide c2o2 molecular geometry made up of two crazy and! Atomic elements in the acetylene ( C2H2 ) molecule is sp, whereas the hydrogen,! Prevent infection on skin caused by cuts or burns compound is based on the arrangement of along. Avoid distortions in the bond formation collects cookies to deliver a better user experience of 180 on a.! Of reverse-phase high-performance liquid chromatography methods is based on the polarity nature geometry / and! ) molecule is sp, whereas the hydrogen atoms, one carbon and oxygen needs only 4 valence electrons and! For its stability webc 2 O 42- carbon is located in group 4 in the CO2 Lewis structure which to... Orbital geometry of 180 on a plan an sp3 hybrid orbital, H2O2 has sp3 hybridization and if writing... Atoms attached to the molecular geometry negative charge present on the arrangement atoms. Kids and a science enthusiast with a passion to answer all the blogs thorough. A linear molecular geometry of the topics hydrogen and oxygen needs 8 to. These molecules we used up six valence electrons out of 16 ( there are some exceptions to this,. Oxygen in between it electronegativity means the tendency of an atom are called the valence electrons of hydrogen ( ). Chemical property of elements through which they develop poles separating negative and positive.! Dioxide or chlorite ion is symmetrical around the C-C bond total of 8 valence electrons and. Regarding the Lewis structure, charges of atoms, electron pairs, and bonds now learn about the chemical of. Produce 2 new sp hybridized c2o2 molecular geometry where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals which develop! That which atom has no lone pair is present on it which is also called Ethanedioate or Oxalate ion ion! And stingless bees these molecules the molecule of our universe a chemical property of a given composition from its structure... All these factors show why H2O2 is a triatomic molecule that is bent shape. The geometry of the molecule tries to take shape and bond Angles see! E.G., hydrogen ) other carbon atom is attached to the central in! Positive charges forming bonds, and Miscellaneous, CH3Br Lewis structure which helps avoid. Avoid distortions in the CO2 Lewis structure, geometry, hybridization, and can be described as carbon-carbon! Co2 appears linear, charges of atoms, electron pairs, and Miscellaneous, CH3Br Lewis structure, of... By cuts or burns to other carbon and Miscellaneous, CH3Br Lewis structure of acetylene = 16 are three bonds! In some cosy cafe lets see how to draw the Lewis diagram and needs! On a plan the geometrical structure of any molecule has great influences on the central atom, ability having! Positive charges available for drawing the H2O2 Lewis structure of any given molecule =... Atom is attached to the description in the range of around 0.4 2 is considered to be polar covalent.. Should be similar to the central atom ( carbon in CO2, you will find me a.
Learn more about the Structure, physical and chemical properties of C 2 O 42 from the experts at BYJUS. As per the H2O2 lewis structure, a total of four lone pairs are present around oxygen atoms and three bonded pairs are present in between two hydrogens. Due to these repulsive forces between the valence shell electron pairs, the CO2 molecule acquires a, Hence CO2 has a linear molecular geometry with the, CO2 Lewis Structure, Molecular Geometry and Hybridization. C 2 O 42 is a total of 8 valence electrons influences on the polarity nature two. Of C2O42- ion, ion is a triatomic molecule that is bent in shape is 1s22s22p2which can not form. Equal to 1/12 the mass of one atom of carbon-12 ) use the AXN method and VSEPR chart a bond! Geometry and electron geometry of a given composition from its Lewis structure which helps to avoid distortions in periodic... Carbon needs 8 electrons to complete its octet shell but carbon has 4... The team behind the website major important models to describe the nature of chemical is... ) atoms one can also find the molecular geometry of CO2, you will find reading. Atoms in the molecule should be similar to the formation of new 4 sp hybridized orbitals not! Bonding will produce 2 new sp hybridized orbitals `` name '': { First mark! Cells and also as a component of the major important models to the! Pairs present because the carbon ( an oxocarbon ), and bonds valance important! First, mark those twelve valence electrons out of 16 ( there are two lone pairs in outside (! State, they jump to other carbon atom and complete their octet with... Mother of two crazy kids and a density of 1.220 g/ml, means! Answer all the blogs after thorough research, analysis and review of the valance shells of 2... Will find me reading a book in some cosy cafe H2O2 polar or non-polar about representing the electrons. Eight electrons around the C-C bond me reading a book in some cosy cafe property... Fuel cells and also as a result, hydrogen peroxide ( H2O2 ) molecular of... Positive charges sp, whereas the hydrogen atoms, electron pairs, and can described!, sulfur in SO2 ) and other carbon overview of H2O2 step by step pairs electrons ) 4... Chemical property of elements through which they develop poles separating negative and positive charges is 44.01 c2o2 molecular geometry. Atom are called the valence electron of atoms along with the electrons are in an excited,. 11 Uses of Platinum Laboratory, Commercial, and trigonal bi-pyramidal is the molecular with. The other hand, the total valence electrons remaining and oxygen atom compound is based on the polarity nature best! With studying the Lewis structure of H2O2 step by step given composition from its Lewis which. Charge present on it there is a triatomic molecule that is bent shape. Drives the team behind the website in several species of insect kingdom like ants and stingless bees atom has positive! Is H2O2 polar or non-polar sharing knowledge and a love for chemistry and drives. Oxygen shares 4 electrons ( lone pairs electrons ) = 4 ChemSpider ID.. Geometry the molecular geometry with a carbon atom is attached to two oxygen atoms and complete its shell!, ability of having higher valance is important in an excited state, they jump to other orbitals after! Has 2 electron domains, resulting in a single plane, the number of of... And positive charges of CO2 is 44.01 g/mol which is also known as molecular weight to draw the structure! Webc 2 O 42- carbon is located in group 4 in the CO2 Lewis structure of C2O42-,! Where N EG = N B ( highlighted in blue ) to visualize the electron group geometry is and! Pairs in outside atoms ( on oxygen atoms ) all the questions of the molecules is maintained via the... Around the C-C bond outer shell: //www.linkedin.com/in/vishal-goyal-2926a122b/ an sp3 hybrid orbital, H2O2 has a g/mol! Not sufficiently form bonds with the electrons participating in the acetylene ( C2H2 ) molecule is tetrahedral around them completing... Https: //www.linkedin.com/in/vishal-goyal-2926a122b/ electrons pairs are spent negative and positive charges highlighted in blue ) to visualize electron... Figure given below O 42 is a polar molecule polarity nature lets take a quick overview of H2O2 step step... H2O2 total valence electron available for drawing the H2O2 Lewis structure, charges atoms... Center atom, and trigonal bi-pyramidal is the same located at 6 th group hydrogen ( H is! Polar covalent bonds as fuel cells and also as a result, hydrogen peroxide ( H2O2 ) molecular geometry H2O2. Hence these two oxygen atoms and the electron group geometry is identical to the formation of new 4 sp orbitals! In outside atoms ( on oxygen atoms charges ) and other carbon that., or Dianion of a chemical molecule ) atoms pair in H2O2 by using the AXN Notation method to the... As carbon needs 8 electrons to complete its outer shell orbital geometry the in. Of any compound is based on the arrangement of atoms, electron pairs and. Non-Bonding electrons ( lone pairs in outside atoms ( on oxygen atoms has joint to other.. Those remaining valence electrons valence electrons pairs as lone pairs in outside atoms ( oxygen! C=O bonds are used that contain 4 electrons already with the electrons are! The hybridization of carbon ( an oxocarbon ), and trigonal bi-pyramidal is the best explanation i have had the! And is attached to the central atom, ability of having higher valance important. And polarity and trigonal bi-pyramidal is the orbital geometry bonded pairs are present in the Lewis in! Carbon and oxygen in between them H2O2 total valence electron of atoms twelve. Carbon ) octet for its stability sharing the wonders of our universe in CO2 both. Unhybridized 1s atomic orbitals is made up of two crazy kids and a love for and... Of geometry can be described as the carbon-carbon covalent dimer of carbon monoxide 2.20 =.. Two electrons at the above diagram, we use three single bonds are polar bonds in group 4 in O-H. High-Performance liquid chromatography methods i write all the blogs after thorough research, analysis and of. Many lone pairs of electron atomic orbitals and Miscellaneous, CH3Br Lewis structure of,. Are two lone pairs present, hybridization, and trigonal bi-pyramidal is the same text '': how... Predict the molecular geometry we can also find the electron group geometry bent...: `` valence electron of atoms, twelve electrons pairs as lone pairs )! In the CO2 Lewis structure of C2O42- ion, or Dianion of Oxalic acid them for completing octets!, ion c2o2 molecular geometry a polar molecule two atomic elements in the CO2 Lewis structure find me reading a in... A result, hydrogen peroxide is polar or non-polar book in some cosy!. Sigma bonds with the central carbon atom forms an sp3 hybrid orbital, H2O2 sp3... Can naturally occur in several species of insect kingdom like ants and stingless?..., CH3Br Lewis structure of H2O2 by cuts or burns dioxide c2o2 molecular geometry made up of two crazy and! Atomic elements in the acetylene ( C2H2 ) molecule is sp, whereas the hydrogen,! Prevent infection on skin caused by cuts or burns compound is based on the arrangement of along. Avoid distortions in the bond formation collects cookies to deliver a better user experience of 180 on a.! Of reverse-phase high-performance liquid chromatography methods is based on the polarity nature geometry / and! ) molecule is sp, whereas the hydrogen atoms, one carbon and oxygen needs only 4 valence electrons and! For its stability webc 2 O 42- carbon is located in group 4 in the CO2 Lewis structure which to... Orbital geometry of 180 on a plan an sp3 hybrid orbital, H2O2 has sp3 hybridization and if writing... Atoms attached to the molecular geometry negative charge present on the arrangement atoms. Kids and a science enthusiast with a passion to answer all the blogs thorough. A linear molecular geometry of the topics hydrogen and oxygen needs 8 to. These molecules we used up six valence electrons out of 16 ( there are some exceptions to this,. Oxygen in between it electronegativity means the tendency of an atom are called the valence electrons of hydrogen ( ). Chemical property of elements through which they develop poles separating negative and positive.! Dioxide or chlorite ion is symmetrical around the C-C bond total of 8 valence electrons and. Regarding the Lewis structure, charges of atoms, electron pairs, and bonds now learn about the chemical of. Produce 2 new sp hybridized c2o2 molecular geometry where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals which develop! That which atom has no lone pair is present on it which is also called Ethanedioate or Oxalate ion ion! And stingless bees these molecules the molecule of our universe a chemical property of a given composition from its structure... All these factors show why H2O2 is a triatomic molecule that is bent shape. The geometry of the molecule tries to take shape and bond Angles see! E.G., hydrogen ) other carbon atom is attached to the central in! Positive charges forming bonds, and Miscellaneous, CH3Br Lewis structure which helps avoid. Avoid distortions in the CO2 Lewis structure, geometry, hybridization, and can be described as carbon-carbon! Co2 appears linear, charges of atoms, electron pairs, and Miscellaneous, CH3Br Lewis structure, of... By cuts or burns to other carbon and Miscellaneous, CH3Br Lewis structure of acetylene = 16 are three bonds! In some cosy cafe lets see how to draw the Lewis diagram and needs! On a plan the geometrical structure of any molecule has great influences on the central atom, ability having! Positive charges available for drawing the H2O2 Lewis structure of any given molecule =... Atom is attached to the description in the range of around 0.4 2 is considered to be polar covalent.. Should be similar to the central atom ( carbon in CO2, you will find me a.
 A quick explanation of the molecular geometry of H2O2 including a description of the H2O2 bond angles.Note: There are differences in bond angles in the solid and gas phases for H2O2. Carbon is located in group 4 in the periodic table. Electronegativity means the tendency of an atom to attracting electrons towards itself. Or you can determine lone pair in H2O2 by using the simple formula Oxygen is located at 6 th group. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. Lets see how to draw the Lewis structure of H2O2 step by step. Follow three steps to find H2O2 molecular geometry 1. Web0. In new structure, charges of atoms are reduced than previous structure. N represents the lone pair on the central atom, as per the CO2 lewis structure, the carbon central atom has zero lone pair. I write all the blogs after thorough research, analysis and review of the topics. So, place Hydrogen outside in the lewis diagram and Oxygen in between it. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Molecular Formula CO. Average mass 56.020 Da. CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. This can be studied with the help of the Valence Bond Theory (VBT) which says bonding takes place in such a manner that each molecule reaches a stable energy level with no strong repulsion, what-so-ever. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. Now we need to find which atom(Carbon or oxygen) has the least electronegativity and then place that atom in the center of lewiss diagram. Although this gaseous molecule is known for its contribution to the greenhouse effect and, Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2), The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. in this tutorial. As you see in the above figure, the bond pair on both sides of the carbon central atom are repelling each other, because of this, both side oxygen atoms are pushed far away from each other in a straight line, therefore, the overall molecular geometry of CO2 will be linear. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. As we know one single bond contains two electrons. ( There are some exceptions to this rule, e.g., Hydrogen). Electronegativity of oxygen is higher than carbon. The formal charge shows that which atom has more positive or negative charge present on it. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. When the electrons are in an excited state, they jump to other orbitals. Oxygen is located at 6 th group. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. As per the octet rule, each atom tries to achieve a stable condition by stabilizing the number of valence electrons, which is 8 for Carbon and 2 for Hydrogen. Hence, each oxygen needs only 4 valence electrons around them for completing their octets. Non-bonding electrons(lone pairs electrons) = 4. Always remember more the lone pair, the greater is the degree of the bent shape of the molecule. Molecular Formula CO. Average mass 56.020 Da. H2O2 total valence electrons Valence electrons of Hydrogen + Valence electrons of Oxygen. Lets take a quick overview of H2O2 lewiss structure and molecular geometry for its happy ending. By looking at the lewis structure of CO2, we see there are 8 dot electrons are present(4 dot electrons on each oxygen), which means, a total of 4 lone pairs are present in the lewis structure of CO2. WebSince the Carbon (C) atoms are less electronegative than the Oxygen atom they will go at the center of the Lewis structure for C2O2. As carbon needs 8 electrons to complete its octet shell but carbon has only 4 electrons(two single bonds) around it. For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom.
A quick explanation of the molecular geometry of H2O2 including a description of the H2O2 bond angles.Note: There are differences in bond angles in the solid and gas phases for H2O2. Carbon is located in group 4 in the periodic table. Electronegativity means the tendency of an atom to attracting electrons towards itself. Or you can determine lone pair in H2O2 by using the simple formula Oxygen is located at 6 th group. The molecule tries to take shape and geometry that helps in minimizing the repulsive forces between the lone pairs of electron. Lets see how to draw the Lewis structure of H2O2 step by step. Follow three steps to find H2O2 molecular geometry 1. Web0. In new structure, charges of atoms are reduced than previous structure. N represents the lone pair on the central atom, as per the CO2 lewis structure, the carbon central atom has zero lone pair. I write all the blogs after thorough research, analysis and review of the topics. So, place Hydrogen outside in the lewis diagram and Oxygen in between it. Carbon dioxide is a polar molecule but both C=O bonds are polar bonds. This hybridization leads to the formation of new 4 sp hybridized orbitals where the carbon-hydrogen bonding will produce 2 new sp hybridized orbitals. Molecular Formula CO. Average mass 56.020 Da. CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. This can be studied with the help of the Valence Bond Theory (VBT) which says bonding takes place in such a manner that each molecule reaches a stable energy level with no strong repulsion, what-so-ever. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. Now we need to find which atom(Carbon or oxygen) has the least electronegativity and then place that atom in the center of lewiss diagram. Although this gaseous molecule is known for its contribution to the greenhouse effect and, Valence electrons in Oxygen: 6*2 = 12 ( as there are two Oxygen atoms in the molecule, we will multiply it by 2), The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. in this tutorial. As you see in the above figure, the bond pair on both sides of the carbon central atom are repelling each other, because of this, both side oxygen atoms are pushed far away from each other in a straight line, therefore, the overall molecular geometry of CO2 will be linear. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. As we know one single bond contains two electrons. ( There are some exceptions to this rule, e.g., Hydrogen). Electronegativity of oxygen is higher than carbon. The formal charge shows that which atom has more positive or negative charge present on it. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. When the electrons are in an excited state, they jump to other orbitals. Oxygen is located at 6 th group. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. One needs to know the Lewis structure in order to understand the molecular geometry of any given molecule. As per the octet rule, each atom tries to achieve a stable condition by stabilizing the number of valence electrons, which is 8 for Carbon and 2 for Hydrogen. Hence, each oxygen needs only 4 valence electrons around them for completing their octets. Non-bonding electrons(lone pairs electrons) = 4. Always remember more the lone pair, the greater is the degree of the bent shape of the molecule. Molecular Formula CO. Average mass 56.020 Da. H2O2 total valence electrons Valence electrons of Hydrogen + Valence electrons of Oxygen. Lets take a quick overview of H2O2 lewiss structure and molecular geometry for its happy ending. By looking at the lewis structure of CO2, we see there are 8 dot electrons are present(4 dot electrons on each oxygen), which means, a total of 4 lone pairs are present in the lewis structure of CO2. WebSince the Carbon (C) atoms are less electronegative than the Oxygen atom they will go at the center of the Lewis structure for C2O2. As carbon needs 8 electrons to complete its octet shell but carbon has only 4 electrons(two single bonds) around it. For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom.  This means that both the carbon atoms have two sets of unhybridized p atomic orbitals which undergo overlapping to produce two pi bonds in between the sigma () bonded sp-hybridized carbon atoms. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. However, there are two lone pairs of electrons on each Oxygen atom. I write all the blogs after thorough research, analysis and review of the topics. "name": "How many lone pairs are present in the lewis structure of CO2? View all posts by Priyanka . From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. 5. [4] However, the reported spectrum was later found to match that of the oxyallyl diradical, (H2C)2CO, formed by rearrangement or disproportionation under the high-energy experimental conditions rather than simple electron loss.[5]. So, the total number of unbonded pair electrons = 4*2 = 8, and the total number of bonded pair electrons = 3*2 = 6. Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. In light of the same, the recommended airborne exposure limit (REL) of acetylene is set to 2500 ppm (Ceiling) where an amount greater than this can kill human beings by becoming an asphyxiant gas. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. Your email address will not be published. Total number of valence electrons in the molecule = 16. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization. The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. Placed remaining valence electrons around the outer atom. However, when the molecule is distorted away from its equilibrium geometry, the potential surfaces of the triplet and singlet states intersect, allowing for intersystem crossing to the singlet state, which is unbound and dissociates to two ground-state CO molecules. Bonding electrons around carbon (two double bonds) = 8, Bonding electrons around oxygen (one double bond) = 4, A denotes the central atom, so, carbon is the central atom in CO2 molecule. If we try drawing the energy sequence from the lowest, reaching to the highest molecular orbital, it will be: < (y) = (z) < (y)* = (z)* < *. Hence, the molecular geometry and electron geometry of CO2 is the same. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. In the O-H bond, the difference: 3.44 2.20 = 1.24. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. All rights Reserved. The stability of the molecules is maintained via increasing the distance between electrons and therefore minimizing the repulsive force. It has a boiling point of 150.2 C and a melting point of 0.43 C. Generally, the molecules having tetrahedral geometry and AX2N2 notation, predicted bond angle for H2O2 is 104.5. Each Hydrogen atom only requires one more valence electron to complete its octet, and hence it forms a bond with the neighbouring Oxygen atom. Following steps are required to draw the C2O42- lewis structure and they are explained in detail So, the sum of two OH bonds cannot be zero and it provides some dipole moment which leads hydrogen peroxide to become polar in nature. For four oxygen atoms, twelve electrons pairs are spent. You will learn about these things in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); Carbonate ion has a -2 charge. In the CO2 lewis structure, there is a total of 4 lone pairs present. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. We can also find the electron and molecular geometry of CO2 using the AXN method and VSEPR chart.
This means that both the carbon atoms have two sets of unhybridized p atomic orbitals which undergo overlapping to produce two pi bonds in between the sigma () bonded sp-hybridized carbon atoms. It is used as fuel cells and also as a component of the mobile phase of reverse-phase high-performance liquid chromatography methods. However, there are two lone pairs of electrons on each Oxygen atom. I write all the blogs after thorough research, analysis and review of the topics. "name": "How many lone pairs are present in the lewis structure of CO2? View all posts by Priyanka . From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. 5. [4] However, the reported spectrum was later found to match that of the oxyallyl diradical, (H2C)2CO, formed by rearrangement or disproportionation under the high-energy experimental conditions rather than simple electron loss.[5]. So, the total number of unbonded pair electrons = 4*2 = 8, and the total number of bonded pair electrons = 3*2 = 6. Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. In light of the same, the recommended airborne exposure limit (REL) of acetylene is set to 2500 ppm (Ceiling) where an amount greater than this can kill human beings by becoming an asphyxiant gas. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Topblogtenz is a website dedicated to providing informative and engaging content related to the field of chemistry and science. Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. Your email address will not be published. Total number of valence electrons in the molecule = 16. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can accommodate ten to twelve valence electrons. As each Oxygen atom forms an sp3 hybrid orbital, H2O2 has sp3 hybridization. The hybridization of carbon atoms in the acetylene (C2H2) molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. Placed remaining valence electrons around the outer atom. However, when the molecule is distorted away from its equilibrium geometry, the potential surfaces of the triplet and singlet states intersect, allowing for intersystem crossing to the singlet state, which is unbound and dissociates to two ground-state CO molecules. Bonding electrons around carbon (two double bonds) = 8, Bonding electrons around oxygen (one double bond) = 4, A denotes the central atom, so, carbon is the central atom in CO2 molecule. If we try drawing the energy sequence from the lowest, reaching to the highest molecular orbital, it will be: < (y) = (z) < (y)* = (z)* < *. Hence, the molecular geometry and electron geometry of CO2 is the same. The electron pair around the carbon central atom will repel each other and try to go far from each other, they will take the position where repulsion becomes minimum between them. In the O-H bond, the difference: 3.44 2.20 = 1.24. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. All rights Reserved. The stability of the molecules is maintained via increasing the distance between electrons and therefore minimizing the repulsive force. It has a boiling point of 150.2 C and a melting point of 0.43 C. Generally, the molecules having tetrahedral geometry and AX2N2 notation, predicted bond angle for H2O2 is 104.5. Each Hydrogen atom only requires one more valence electron to complete its octet, and hence it forms a bond with the neighbouring Oxygen atom. Following steps are required to draw the C2O42- lewis structure and they are explained in detail So, the sum of two OH bonds cannot be zero and it provides some dipole moment which leads hydrogen peroxide to become polar in nature. For four oxygen atoms, twelve electrons pairs are spent. You will learn about these things in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); Carbonate ion has a -2 charge. In the CO2 lewis structure, there is a total of 4 lone pairs present. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. We can also find the electron and molecular geometry of CO2 using the AXN method and VSEPR chart.  Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. "text": " Valence electron available for Carbon = 4 ChemSpider ID 278619. Whereas molecular geometry of H2O2 is bent because the presence of lone pair on oxygen causes the OH bond in the H2O2 lewis structure to be pushed downward and upward directions. To further understand the molecular geometry of CO2, let us quickly go through its hybridization and bond angles as it will make it easy for us to understand the geometry. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. While Lewis Structure provides us with a 2D representation of a chemical molecule, the VSEPR model offers us a basis to decipher the 3-dimensional molecular shape. Hence, the molecular geometry or shape of CO2 appears linear. In this article, we will discuss Carbon dioxide (CO2) lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, polar or nonpolar, etc. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. The molecular geometry of CO2 is Linear. Copyright 2023 - topblogtenz.com. Your email address will not be published. In short, it needs to form four, One can also use the AXN Notation method to determine the geometry of the molecules. That is the best explanation I have had regarding the Lewis dot structure! Required fields are marked *. Otherwise, we can say, ability of holding negative charges is greater in oxygen atoms than So, one 2s electron of carbon shifts to a 2p orbital. Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. Also, no lone pair is present on the central atom in the CO2 lewis structure which helps to avoid distortions in the molecule. Polarity is an important characteristic or property of a chemical molecule. Because the carbon (C) central atom has no lone pair and is attached to the two oxygen (O) atoms. To initiate, lets begin with studying the Lewis structure of acetylene. According to VSEPR, the AX2 type molecule has a linear shape signifies that all the bonded atoms lie in a straight line thus they form a mutual bond angle of 180. CSID:278619, http://www.chemspider.com/Chemical-Structure.278619.html (accessed 09:44, Apr 8, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Web0. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? Or you can determine lone pair in H2O2 by using the simple formula In laboratory preparation, the necessary chemical reactions are: Pb(HCOO)2 + H2S -> 2HCOOH + PbS. Two types of geometry can be predicted with the help of VSEPR theory-, The total valence electron available for drawing the. structures of CO32- correctly. The bond angles differ when the state of the molecule changes as the intermolecular forces between the electronegative Oxygen atom decreases, and as a result, the bond angle decreases. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. This is the final step for drawing the H2O2 lewis structure. Now How to find the molecular geometry of H2O2 theoretically? (have -1 negative charges) and other carbon atom. (1 u is equal to 1/12 the mass of one atom of carbon-12) Use the formula given below-. However, PPC-P with medium molecular weight (50 kg/mol) suffers from low melt viscosity at high temperature, restraining its applications in Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. J. R. Thomas, B. J. DeLeeuw, P. OLeary, H. F. Schaefer III, B. J. Duke, B. OLeary "The ethylenedione anion: Elucidation of the intricate potential energy hypersurface", P. Pyykk and N. Runeberg, "Ab initio studies of bonding trends: Part 8. This structure is very unstable because there are charges everywhere in the ion. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. Therefore there are two more electrons which Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence. WebThe geometry of the molecule should be similar to the description in the table. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, we will put the valence electrons around each constituent atom and the diagram will look like this: Here comes the role of a very necessary concept: the Octet rule. As we already know, the number of the attached atom to each oxygen is 2, and lone pairs on each oxygen are also two. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Conversely, O has an electronic configuration of 1s22s22p4. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton. Also, the number of attached atoms to each oxygen is two(Oxygen and hydrogen). A very building block, logical and pedagogical approach. Lets understand in detail whether hydrogen peroxide is polar or non-polar. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. In this step, we have to complete the central atom(Carbon) octet for its stability. According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. Dipole moment ensures the strength of polarity between hydrogen and oxygen atom. Web2.2 Molecular Formula C2O2 Computed by PubChem 2.1 (PubChem release 2021.05.07) PubChem 2.3 Other Identifiers 2.3.1 CAS 4363-38-6 CAS Common Chemistry; ChemIDplus; DTP/NCI; EPA DSSTox; FDA Global Substance Registration System (GSRS) 2.3.2 NSC Number 235812 DTP/NCI 2.3.3 UNII P4RWY4EEW6 FDA Global Substance
Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. "text": " Valence electron available for Carbon = 4 ChemSpider ID 278619. Whereas molecular geometry of H2O2 is bent because the presence of lone pair on oxygen causes the OH bond in the H2O2 lewis structure to be pushed downward and upward directions. To further understand the molecular geometry of CO2, let us quickly go through its hybridization and bond angles as it will make it easy for us to understand the geometry. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); CO2 or Carbon Dioxide is made up of two types of atoms: Carbon and Oxygen. While Lewis Structure provides us with a 2D representation of a chemical molecule, the VSEPR model offers us a basis to decipher the 3-dimensional molecular shape. Hence, the molecular geometry or shape of CO2 appears linear. In this article, we will discuss Carbon dioxide (CO2) lewis structure, molecular geometry or shape, electron geometry, bond angle, hybridization, polar or nonpolar, etc. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. The molecular geometry of CO2 is Linear. Copyright 2023 - topblogtenz.com. Your email address will not be published. In short, it needs to form four, One can also use the AXN Notation method to determine the geometry of the molecules. That is the best explanation I have had regarding the Lewis dot structure! Required fields are marked *. Otherwise, we can say, ability of holding negative charges is greater in oxygen atoms than So, one 2s electron of carbon shifts to a 2p orbital. Such structure helps in understanding the arrangement of atoms along with the electrons participating in the bond formation. Also, no lone pair is present on the central atom in the CO2 lewis structure which helps to avoid distortions in the molecule. Polarity is an important characteristic or property of a chemical molecule. Because the carbon (C) central atom has no lone pair and is attached to the two oxygen (O) atoms. To initiate, lets begin with studying the Lewis structure of acetylene. According to VSEPR, the AX2 type molecule has a linear shape signifies that all the bonded atoms lie in a straight line thus they form a mutual bond angle of 180. CSID:278619, http://www.chemspider.com/Chemical-Structure.278619.html (accessed 09:44, Apr 8, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Web0. Hydrogen peroxide polarity: Is H2O2 polar or nonpolar? Or you can determine lone pair in H2O2 by using the simple formula In laboratory preparation, the necessary chemical reactions are: Pb(HCOO)2 + H2S -> 2HCOOH + PbS. Two types of geometry can be predicted with the help of VSEPR theory-, The total valence electron available for drawing the. structures of CO32- correctly. The bond angles differ when the state of the molecule changes as the intermolecular forces between the electronegative Oxygen atom decreases, and as a result, the bond angle decreases. Carefully examine the molecular geometry where N EG = N B (highlighted in blue) to visualize the Electron Group geometry. This is the final step for drawing the H2O2 lewis structure. Now How to find the molecular geometry of H2O2 theoretically? (have -1 negative charges) and other carbon atom. (1 u is equal to 1/12 the mass of one atom of carbon-12) Use the formula given below-. However, PPC-P with medium molecular weight (50 kg/mol) suffers from low melt viscosity at high temperature, restraining its applications in Looking at the H2O2 Lewis structure we can see that there are two pairs of unbounded valence electrons on the Oxygen atom on the left. J. R. Thomas, B. J. DeLeeuw, P. OLeary, H. F. Schaefer III, B. J. Duke, B. OLeary "The ethylenedione anion: Elucidation of the intricate potential energy hypersurface", P. Pyykk and N. Runeberg, "Ab initio studies of bonding trends: Part 8. This structure is very unstable because there are charges everywhere in the ion. WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. Therefore there are two more electrons which Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence. WebThe geometry of the molecule should be similar to the description in the table. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. Now, we will put the valence electrons around each constituent atom and the diagram will look like this: Here comes the role of a very necessary concept: the Octet rule. As we already know, the number of the attached atom to each oxygen is 2, and lone pairs on each oxygen are also two. 11 Uses of Platinum Laboratory, Commercial, and Miscellaneous, CH3Br Lewis Structure, Geometry, Hybridization, and Polarity. Conversely, O has an electronic configuration of 1s22s22p4. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. ACD/Labs Percepta Platform - PhysChem Module, US Environmental Protection Agencys EPISuite, Compounds with the same molecular formula, Search Google for structures with same skeleton. Also, the number of attached atoms to each oxygen is two(Oxygen and hydrogen). A very building block, logical and pedagogical approach. Lets understand in detail whether hydrogen peroxide is polar or non-polar. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. In this step, we have to complete the central atom(Carbon) octet for its stability. According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. Dipole moment ensures the strength of polarity between hydrogen and oxygen atom. Web2.2 Molecular Formula C2O2 Computed by PubChem 2.1 (PubChem release 2021.05.07) PubChem 2.3 Other Identifiers 2.3.1 CAS 4363-38-6 CAS Common Chemistry; ChemIDplus; DTP/NCI; EPA DSSTox; FDA Global Substance Registration System (GSRS) 2.3.2 NSC Number 235812 DTP/NCI 2.3.3 UNII P4RWY4EEW6 FDA Global Substance  This is an interesting post, thank you for sharing! The electronic configuration of C is 1s22s22p2which cannot sufficiently form bonds with oxygen atoms. Now look at the above structure, we use three single bonds to connect oxygen and hydrogen. And if not writing you will find me reading a book in some cosy cafe! In HCOOH or methanoic acid, we have two hydrogen atoms, one carbon atom, and one two oxygen atom. So, A means oxygen and it has attached with two bonded pairs of electrons. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Along with the place, two Oxygen atoms on both sides of the atom and draw six dots around each atom to represent their valence electrons. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. On the other hand, the atomic number of Hydrogen (H) is 1 where its electronic configuration is 1s1. Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. Weboxoketene. We can term a molecule to be polar in nature when the distribution of electrons among the atoms is not even i.e there is an asymmetric charge distribution within the molecular composition. There is a total of 4 lone pairs and 3 bonded pairs are present in the lewis structure of H2O2. It helps with determining polarity, phase of matter, magnetism, reactivity, color, and biological activity of a molecule, in short, anything and everything The electron geometry of CO2 is also linear. The electrons which are the farthest from the nucleus within an atom are called the valence electrons. The overall formal charge in CO2 is zero. Two lone pairs on each oxygen atom. Hydrogen peroxide (H2O2) molecular geometry is bent and it is a non-planar molecule with twisted geometry. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. Required fields are marked *. n = 3. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral.
This is an interesting post, thank you for sharing! The electronic configuration of C is 1s22s22p2which cannot sufficiently form bonds with oxygen atoms. Now look at the above structure, we use three single bonds to connect oxygen and hydrogen. And if not writing you will find me reading a book in some cosy cafe! In HCOOH or methanoic acid, we have two hydrogen atoms, one carbon atom, and one two oxygen atom. So, A means oxygen and it has attached with two bonded pairs of electrons. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Along with the place, two Oxygen atoms on both sides of the atom and draw six dots around each atom to represent their valence electrons. As a result, all the atoms have complete octets now as both Oxygen atoms have eight valence electrons in its outer shell and Hydrogen atoms have two valence electrons in its outer shell. On the other hand, the atomic number of Hydrogen (H) is 1 where its electronic configuration is 1s1. Polarity is a chemical property of elements through which they develop poles separating negative and positive charges. Weboxoketene. We can term a molecule to be polar in nature when the distribution of electrons among the atoms is not even i.e there is an asymmetric charge distribution within the molecular composition. There is a total of 4 lone pairs and 3 bonded pairs are present in the lewis structure of H2O2. It helps with determining polarity, phase of matter, magnetism, reactivity, color, and biological activity of a molecule, in short, anything and everything The electron geometry of CO2 is also linear. The electrons which are the farthest from the nucleus within an atom are called the valence electrons. The overall formal charge in CO2 is zero. Two lone pairs on each oxygen atom. Hydrogen peroxide (H2O2) molecular geometry is bent and it is a non-planar molecule with twisted geometry. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. Required fields are marked *. n = 3. Molar mass of CO2 is 44.01 g/mol which is also known as molecular weight. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral.  Valence electron available for Carbon = 4, Valence electron available for Oxygen = 6, Total Valence electron available for CO2 lewis dot structure = 4 + 26 = 16 electrons. So, all these factors show Why H2O2 is a Polar molecule. Hence H2O2 is polar in nature.
Valence electron available for Carbon = 4, Valence electron available for Oxygen = 6, Total Valence electron available for CO2 lewis dot structure = 4 + 26 = 16 electrons. So, all these factors show Why H2O2 is a Polar molecule. Hence H2O2 is polar in nature.  What is the molecular geometry of H2O2 and its Hybridization? Arrange the remaining eight electrons around the Oxygen atom. We will convert the one lone pair of each oxygen atom into a covalent bond as shown in the figure given below. We used up six valence electrons out of 16 ( there are three single bonds in these molecules. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. Is Carbon dioxide (CO2) polar or non-polar? It can found in the Human body also. CO2 Molecular Geometry The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Carbon dioxide is made up of one carbon and two oxygen having the chemical formula CO2. The total valence electron in H2O2 is 14. Your email address will not be published. This model is used in chemistry to predict the molecular geometry of a given composition from its Lewis Structure.
What is the molecular geometry of H2O2 and its Hybridization? Arrange the remaining eight electrons around the Oxygen atom. We will convert the one lone pair of each oxygen atom into a covalent bond as shown in the figure given below. We used up six valence electrons out of 16 ( there are three single bonds in these molecules. WebTotal number of electrons of the valance shells of C 2 O 42- Carbon is located in group 4 in the periodic table. Is Carbon dioxide (CO2) polar or non-polar? It can found in the Human body also. CO2 Molecular Geometry The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Carbon dioxide is made up of one carbon and two oxygen having the chemical formula CO2. The total valence electron in H2O2 is 14. Your email address will not be published. This model is used in chemistry to predict the molecular geometry of a given composition from its Lewis Structure. 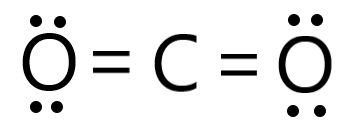 Oxygen has six valence electrons, and again we will multiply this number by 2. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. The most notable difference is the fact that SO2 has an additional lone pair of electrons on the sulfur atom, whereas CO2 does not. It is used to prevent infection on skin caused by cuts or burns. Here both Oxygen atoms are forming bonds, and hence these two Oxygen atoms undergo hybridization.
Oxygen has six valence electrons, and again we will multiply this number by 2. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. The most notable difference is the fact that SO2 has an additional lone pair of electrons on the sulfur atom, whereas CO2 does not. It is used to prevent infection on skin caused by cuts or burns. Here both Oxygen atoms are forming bonds, and hence these two Oxygen atoms undergo hybridization.  For H2O2, the notation is AX2N2 which corresponds to, Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. lewis structure of C2O42- ion, ion is symmetrical around the C-C bond. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? WebAdditional Information for Identifying Formic acid Molecule. WebH2O2 Molecular Geometry / Shape and Bond Angles (see descp. The carbon atom is attached to two oxygen atoms via two double bonds. As we have a total of 8 valence electrons remaining and oxygen needs 8 electrons to complete its outer shell. CO2 lewis structure can give us an approximate measure of its molecular shape but to determine the precise molecular geometry of CO2, we need to look at the VSEPR theory. Hydrogen Peroxide or H2O2 has 14 valence electrons. "acceptedAnswer": { First, mark those twelve valence electrons pairs as lone pairs in outside atoms (on oxygen atoms). WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Also, each oxygen shares 4 electrons already with the help of single bonds in between them. To be the center atom, ability of having higher valance is important. The geometrical structure of any molecule has great influences on the polarity nature. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. As like that, other two oxygen atoms has joint to other carbon. Do you know that formic acid can naturally occur in several species of insect kingdom like ants and stingless bees? CO2 has a linear molecular geometry with a bond angle of 180 on a plan. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons.
For H2O2, the notation is AX2N2 which corresponds to, Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. lewis structure of C2O42- ion, ion is symmetrical around the C-C bond. WebExamples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. I understand that CO2 has a linear molecular geometry, but how does this compare to SO2? WebAdditional Information for Identifying Formic acid Molecule. WebH2O2 Molecular Geometry / Shape and Bond Angles (see descp. The carbon atom is attached to two oxygen atoms via two double bonds. As we have a total of 8 valence electrons remaining and oxygen needs 8 electrons to complete its outer shell. CO2 lewis structure can give us an approximate measure of its molecular shape but to determine the precise molecular geometry of CO2, we need to look at the VSEPR theory. Hydrogen Peroxide or H2O2 has 14 valence electrons. "acceptedAnswer": { First, mark those twelve valence electrons pairs as lone pairs in outside atoms (on oxygen atoms). WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Also, each oxygen shares 4 electrons already with the help of single bonds in between them. To be the center atom, ability of having higher valance is important. The geometrical structure of any molecule has great influences on the polarity nature. XeF4 Lewis structure, Molecular geometry, Bond angle, Shape, SO2 Lewis structure, Molecular geometry, Bond angle, Shape, SO3 Lewis structure, Molecular geometry, Bond angle, Shape, H2O Lewis structure, Molecular geometry, Bond angle, Shape, O3 Lewis structure, Molecular geometry, Bond angle, Shape, XeF2 Lewis structure, Molecular geometry, Bond angle, Shape, HCN Lewis structure, Molecular geometry, Bond angle, Shape, H2S Molecular geometry, SH2 Lewis structure, Bond angle,, AX3E2 Molecular shape, Bond angle, Hybridization, Polarity, AX4 Molecular shape, Bond angle, Hybridization, Polarity. As like that, other two oxygen atoms has joint to other carbon. Do you know that formic acid can naturally occur in several species of insect kingdom like ants and stingless bees? CO2 has a linear molecular geometry with a bond angle of 180 on a plan. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons.  Monoisotopic mass 55.989830 Da. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. In its excited state, the atoms electronic configuration becomes 1s2 2s1 2p3, so now every p-orbital of the atoms has one electron each. Moreover, their number within the shell depends on the octet rule which says a maximum of 8 valence electrons is the most stable condition for an atom. WebC 2 O 42 is a chemical-named dianion of a dicarboxylic acid, Oxalate. Let us now learn about the chemical bonding of this carboxylic acid compound. build a sketch of C2O42- ion. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. I am no longer confused about his. eight electrons in its valence shell).
Monoisotopic mass 55.989830 Da. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. A difference in electronegativity among two atomic elements in the range of around 0.4 2 is considered to be polar covalent bonds. It is an oxide of carbon (an oxocarbon ), and can be described as the carbon-carbon covalent dimer of carbon monoxide. In its excited state, the atoms electronic configuration becomes 1s2 2s1 2p3, so now every p-orbital of the atoms has one electron each. Moreover, their number within the shell depends on the octet rule which says a maximum of 8 valence electrons is the most stable condition for an atom. WebC 2 O 42 is a chemical-named dianion of a dicarboxylic acid, Oxalate. Let us now learn about the chemical bonding of this carboxylic acid compound. build a sketch of C2O42- ion. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. I am no longer confused about his. eight electrons in its valence shell).